水分解
水分解技术的特点是使用化学反应来将水(H2O)分离成氧和氢。有效的水分解可以使用多种不同的技术,包括电解、光合作用、光电化学、光催化、辐射分解、光生物学和热分解来达成。最新的研究成果侧重于使用纳米粒子和薄膜催化剂在较低反应温度下分解水,故而高度共形性原子层沉积 (ALD)已被证明 对于优化细胞效率至关重要。经济高效的水分解是氢生成作为替代能源的关键组件。本领域的研究将调查并测试过渡到氢经济的可行性。
水的光氧化
原子层沉积 (ALD) 技术可用于为晶片分解应用创建高表面积结构。研制了用于水的光氧化(水分解)的高表面积导电透明框架材料。
利用曝光模式技术沉积使用 Savannah® S200 的原子层沉积 (ALD) 膜,在反蛋白结构上用于 ITO 和 Fe2O3 以产生高表面积纳米结构。
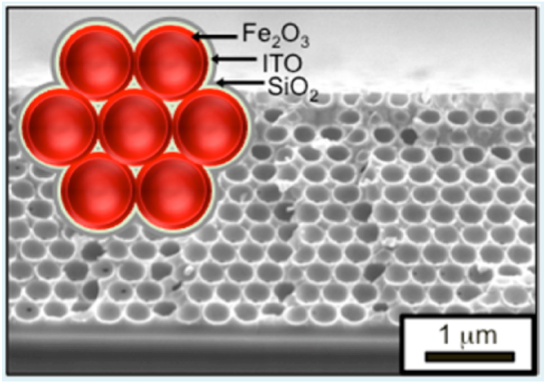
高比表面积、透明且导电的框架用于水的光氧化。通过原子层沉积将氧化铁 Fe2O3、ITO 和 SiO2 沉积在反向支架结构上。参考:Riha, S. C, et al.. Acs Appl Mater Inter 5, 360–367 (2013).
薄膜
- 使用 InCp、TDMASn 和 O3 的铟锡氧化物 (ITO)
- 使用 FeCp2 和 O3 的二茂铁 (Fe2O3)
结果
- 使用原子层沉积 (ALD) 技术为水分解创建高度透明、大表面积纳米层压板叠堆。
- 水氧化的发生改变了 -200 mV,光电流改变了 1.53 V,相比之下可逆氢电极则为三倍。(与平光阳极相比)。
在 Fe2O3 中通过 Ti 替代物进行绿光水氧化
Ti 合金用于改善超薄(6 nm 厚)赤铁矿转化效率,特别是绿光子( 500 – 600 nm)产生的孔收集效率。Savannah® S200 用于沉积本研究中的 TiO2 和 Fe2O3 膜。
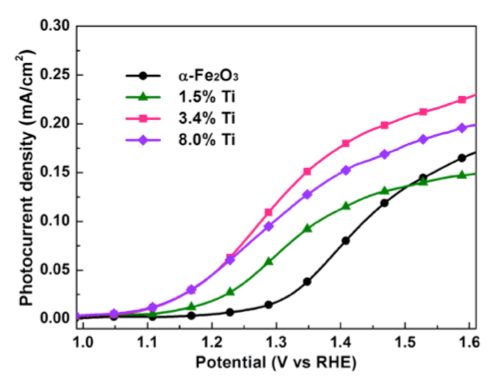
Fe2O3 的钛合金用来提高水的光电化学氧化的催化利用率 。参考:Kim, D. W. et al. Greenlighting Photoelectrochemical Oxidation of Water by Iron Oxide. ACS Nano 141203161851003 (2014).
结果
- 超薄 Fe2O3 中的 Ti 替代物可延长表面局部孔的寿命。
- 观察到 Ti 替代物膜的光电电流性能增加。
- 观察到增强的吸收光电流效率 (APCE),尤其是在 500 – 600 nm 范围。
- 光学吸收未观察到变化。
参考 – 最近在 Veeco CNT 原子层沉积 (ALD)平台上完成的出版物
- Dias, P. et al. Transparent Cuprous Oxide Photocathode Enabling a Stacked Tandem Cell for Unbiased Water Splitting. Adv. Energy Mater. n/a–n/a (2015). doi:10.1002/aenm.201501537
- Luo, J. et al. Targeting Ideal Dual‐Absorber Tandem Water Splitting Using Perovskite Photovoltaics and CuInxGa1‐xSe2 Photocathodes. Adv. Energy Mater. (2015). doi:10.1002/aenm.201501520
- Landsmann, S. et al. Design Guidelines for High-Performance Particle-Based Photoanodes for Water Splitting: Lanthanum Titanium Oxynitride as a Model. ChemSusChem n/a–n/a (2015). doi:10.1002/cssc.201500830
- Barreca, D. et al. Fe2O3–TiO2 Nano‐heterostructure Photoanodes for Highly Efficient Solar Water Oxidation. Advanced Materials Interfaces n/a–n/a (2015). doi:10.1002/admi.201500313
- Michaux, K. E. et al. Visible Photoelectrochemical Water Splitting Based on a Ru(II) Polypyridyl Chromophore and Iridium Oxide Nanoparticle Catalyst. The Journal of … (2015). doi:10.1021/acs.jpcc.5b05711
- Luo, J. et al. Solution Transformation of Cu2O into CuInS2 for Solar Water Splitting. Nano Lett 15, 1395–1402 (2015).
- Schoen, D. T., Atre, A. C., García-Etxarri, A., Dionne, J. A. & Brongersma, M. L. Probing Complex Reflection Coefficients in One-Dimensional Surface Plasmon Polariton Waveguides and Cavities Using STEM EELS. Nano Lett 15, 1–8 (2014).
- Kim, D. W. et al. Greenlighting Photoelectrochemical Oxidation of Water by Iron Oxide. ACS Nano 141203161851003 (2014). doi:10.1021/nn503869n
- Azevedo, J. et al. On the stability enhancement of cuprous oxide water splitting photocathodes by low temperature steam annealing. Energy Environ. Sci. 7, 4044–4052 (2014).
- Yang, X. et al. Improving Hematite-based Photoelectrochemical Water Splitting with Ultrathin TiO 2by Atomic Layer Deposition. Acs Appl Mater Inter 140804065734002 (2014). doi:10.1021/am500948t
- Klahr, B. & Hamann, T. Water Oxidation on Hematite Photoelectrodes: Insight into the Nature of Surface States through In Situ Spectroelectrochemistry. J. Phys. Chem. C 118, 10393–10399 (2014).
- Zandi, O., Beardslee, J. A. & Hamann, T. Substrate Dependent Water Splitting with Ultrathin α-Fe 2O 3Electrodes. J. Phys. Chem. C 140501145249004 (2014). doi:10.1021/jp4116657
- Tilley, D. S. & Grätzel, M. Ruthenium Oxide Hydrogen Evolution Catalysis on Composite Cuprous Oxide Water-Splitting Photocathodes. Advanced Functional … 1–9 (2014). doi:10.1002/adfm201301106
- Yang, X., Du, C., Liu, R., Xie, J. & Wang, D. Balancing photovoltage generation and charge-transfer enhancement for catalyst-decorated photoelectrochemical water splitting: A case study of the hematite/MnOx combination. Journal of Catalysis 304, 86–91 (2013)。
- Liu, M., Nam, C.-Y., Black, C. T., Kamcev, J. & Zhang, L. Enhancing Water Splitting Activity and Chemical Stability of Zinc Oxide Nanowire Photoanodes with Ultrathin Titania Shells. J. Phys. Chem. C 130610143036005 (2013). doi:10.1021/jp404032p
- Riha, S. C. et al. Atomic Layer Deposition of a Sub-Monolayer Catalyst for the Enhanced Photoelectrochemical Performance of Water Oxidation with Hematite. ACS Nano 130212010712000 (2013). doi:10.1021/nn305639z
- Riha, S. C., DeVries Vermeer, M. J., Pellin, M. J., Hupp, J. T. & Martinson, A. B. F. Hematite-based Photo-oxidation of Water Using Transparent Distributed Current Collectors. Acs Appl Mater Inter 5, 360–367 (2013).
- Klug, J. A. et al. Low temperature atomic layer deposition of highly photoactive hematite using iron(iii) chloride and water. J. Mater. Chem. A 1, 11607 (2013).
- Maijenburg, A. W. et al. Electrochemical synthesis of coaxial TiO2-Ag nanowires and their application in photocatalytic water splitting. J. Mater. Chem. A (2013). doi:10.1039/c3ta14551d
- Klahr, B., Gimenez, S., Fabregat-Santiago, F., Bisquert, J. & Hamann, T. W. Photoelectrochemical and Impedance Spectroscopic Investigation of Water Oxidation with ‘Co–Pi’-Coated Hematite Electrodes. J Am Chem Soc 134, 16693–16700 (2012).
- Paracchino, A. et al. Ultrathin films on copper(I) oxide water splitting photocathodes: a study on performance and stability. Energy & Environmental Science 5, 8673–8681 (2012)。
- Klahr, B., Gimenez, S., Fabregat-Santiago, F., Bisquert, J. & Hamann, T. W. Electrochemical and photoelectrochemical investigation of water oxidation with hematite electrodes. Energy & Environmental Science 5, 7626–7636 (2012)。
- Klahr, B. M., Martinson, A. B. F. & Hamann, T. W. Photoelectrochemical Investigation of Ultrathin Film Iron Oxide Solar Cells Prepared by Atomic Layer Deposition. Langmuir 27, 461–468 (2011).
- Liu, R. et al. Water Splitting by Tungsten Oxide Prepared by Atomic Layer Deposition and Decorated with an Oxygen-Evolving Catalyst. Angew. Chem. Int. Ed. 50, 499–502 (2010).